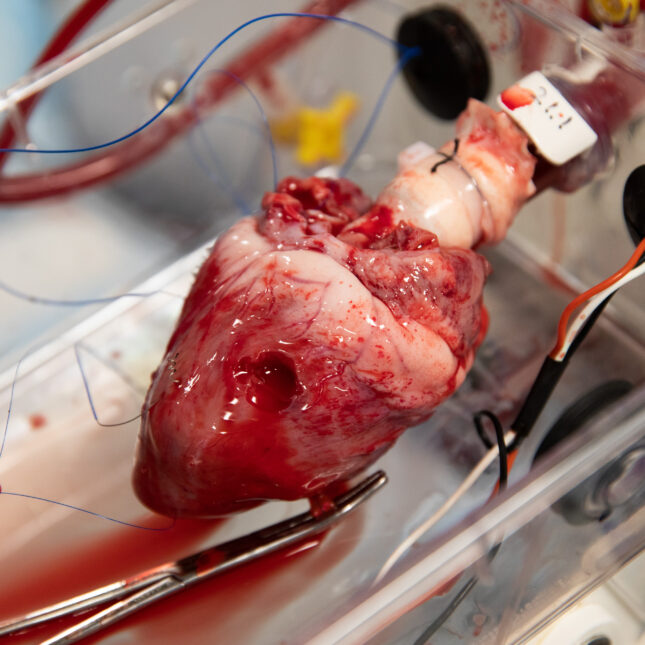
It seems like organized chaos. Five lab members move around a room the size of a galley kitchen. On this day, three high school students also squeeze into the medical lab, closely peering at a pig heart barely beating in a box. Tubes connected to the heart from a rhythmic, speaker-like pump push warm red blood cells through its chambers. It looks like a scene out of Frankenstein.
The heart, which had been kept cold in a liquid bath for about six hours before it was re-animated, appears to be pumping. But not as vigorously as it should. Only the right side of the heart is working, while the left side is still. One of the lab members is massaging the heart to coax the left side into working. She then injects epinephrine into the heart. Eventually, she tries using paddles to shock the heart. After almost two hours, the team calls it. The experiment did not work.
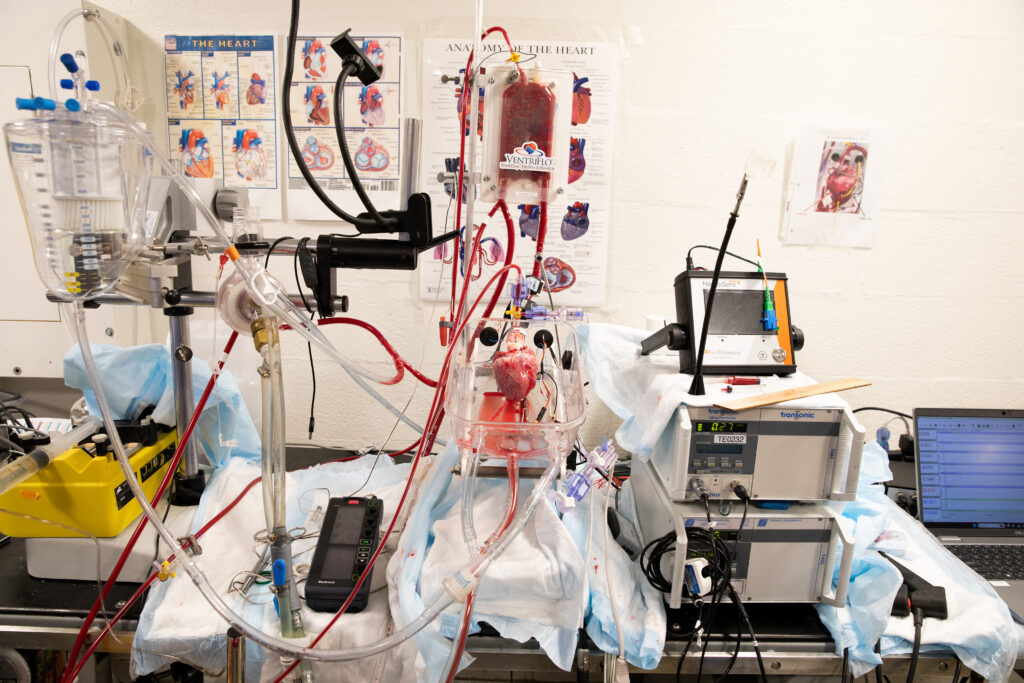
At Massachusetts General Hospital, a trio of medical researchers and a large band of assistants are forging a new path aimed at making more and more hearts viable and available for transplantation. Their focus is on improving a technique known as organ perfusion — taking an organ that has been kept cold for several hours and then reviving it, maintaining the organ outside of the body in a box, and pumping it with blood to keep the tissues alive.
The technology for machine organ perfusion has been around since the 1960s, but the focus has mainly been on kidneys, livers, and later lungs. These organs are able to last outside of the body for a lot longer than hearts.
Early heart transplants involved chilling donated hearts before transplantation, and since the early 1980s some rudimentary form of perfusion, combined with chilling, has been used. With transplants, time is the enemy. Cold has been a way of slowing down time. But too cold for too long and cells start to shut down and die. In the past, hearts from deceased donors couldn’t be kept on ice for more than four hours before the damage started. Now, with new chilling technologies, the promise of perfusion and organ preservation is to push that window from seven to 12 hours to days.
At the MGH Thoracic Organ Ex Vivo Perfusion Lab, the objective is to make warm and cold perfusion technology more sensitive and sophisticated in order to ensure a steady and growing supply of hearts for transplant. Of the 100,000 patients on the waitlist for organs in the U.S., about 3,500 are waiting for hearts, and 5,000 people are added to the list annually — with many of them dying before a suitable heart becomes available. “The reality of it is …we don’t have enough donors,” said Dominic Emerson, a cardiac transplant surgeon and research scientist at Cedars-Sinai in California. Emerson co-runs an organ perfusion lab at Cedars-Sinai with colleague Pedro Catarino, where they are also experimenting with new methods for increasing the donor pool.
Cardiac surgeon Ali Rabi, one of the leaders of the the Mass General team, is researching ways to optimize perfused hearts for transplantation, but he could not do that without the help of his fellow primary investigators Asishana Osho — also a cardiac surgeon — and Shannon Tessier, a researcher in engineering in medicine and surgery. Osho focuses on ways that hearts, especially those donated from patients after circulatory death, known as DCD donors, could be enhanced before transplant. (Most hearts are extracted from brain-dead donors.) Tessier, meanwhile, focuses exclusively on applying the science of hibernation and suspended animation to organs that are being held for transplantation, to prevent them from being injured in the process.
“You know what they say about captains of a ship, but these are three captains that work well together, they actually really collaborate in a very productive way,” said Doug Vincent, a mechanical engineer and the CEO of VentriFlo, which is the system that the lab uses to perfuse the hearts. VentriFlo has been used in the Dynamics Laboratory at the Cleveland Clinic, at Dartmouth-Hitchcock Medical Center, John Fraser’s lab in Brisbane, Australia, and in Robert Bartlett’s Extracorporeal Life Support Laboratory at the University of Michigan.
For Rabi, it made sense for the three researchers to combine their efforts. “I think it was a natural sort of extension of my expertise and our expertise to get [Tessier] involved in the project,” he said. When he learned of her work in maintaining hearts in cold temperatures, he started batting ideas for collaboration around with her. Osho, for his part, knew that somehow he would work with Rabi, whom he’s known for 10 years, and finds their knowledge complementary. Tessier, who just joined the lab three years ago and is not a physician, has helped garner numerous research grants for the lab.
Since December 2022, the lab leaders have used their elaborate perfusion technology on about 68 hearts — 57 from pigs and 11 from patients whose organs were deemed unusable for transplantation. Lab members have been able to reanimate a pig heart and keep it beating for four hours after it sat in cold storage for 24 hours, while another pig heart was kept beating for 12 hours while it was perfused. At Bartlett’s ECLS lab at the University of Michigan, they were able to get similar results, but by actively perfusing a pig heart for 24 hours instead of leaving it in cold storage.
And Mass General’s lab members were able to reanimate a human heart for six hours even though it had been on ice for five to six hours. After the hearts were perfused, the tissue and the perfusate or perfusion liquid were analyzed and other data were collected including pressure, flow, and ultrasound to see how the heart contracted.
For now, the trio isn’t ready to release any data. The group presented abstracts at this year’s International Society for Heart and Lung Transplantation conference in April and they have multiple manuscripts awaiting publication in different journals, including Transplantation Direct. The researchers’ ultimate goal is to standardize the perfusion process so that more hospitals and transplant centers can use the technique, and that should mean an increase in the total number of hearts available for patients in need.

‘Optimizing hearts’
In solid organ transplantation, there are a couple of goals that would be the holy grail of transplantation research, according to Emerson. One is finding a way for transplanted organs to evade rejection; the other is figuring out how to sustain organs outside of donors and recipients for longer periods of time and to rehabilitate them.
“I’ve been interested in trying to optimize the hearts outside of the body before we put them in. The goal is not just to, you know, recover and transplant hearts that are functioning OK, but also to optimize the function of the hearts that are not OK to start with,” said Rabi.
At Mass General, he’s been exploring a few ideas for how to do that. One way of “optimizing” a heart that might not be functioning well is to inject or bathe the heart in a solution with metabolites, then measure the heart’s response to those metabolites. Another focus is to create therapies using siRNA, or small interfering pieces of RNA that block the cascade of signals heart cells send out when they’re deprived of oxygen, that can lead to ischemia or other injury.
“You can think of them like little off switches or blocking packets,” Emerson said. “And if you could switch some of those off, you can decide, ‘right now I just need to turn them off for the next few hours while [the heart’s] outside the body.’ Then you could prevent some of the damage from occurring during the transfer process. It’s a very bright, simple way to address this. I think it’s a fantastic idea,” Emerson said.

This research at Mass General Hospital started towards the end of 2022, after physician-scientists wanted to improve the way that hearts were perfused.
Since March 2023, Rabi’s work has developed to where it is now: pushing the envelope on how long hearts can be perfused. But if it weren’t for his friendship with Osho, which began when they met as interns at this same hospital a decade ago, Rabi might never have found his way to working on hearts at all.
“When I came to surgery here, I wanted to do abdominal surgery, but over time … I actually got more and interested in cardiac surgery,” Rabi said.
Rabi, 40, was born in Iran, the child of a pediatrician mother and OB-GYN father. He didn’t start out wanting to go into medicine, despite the pedigree; after moving with his family to Canada, he did his undergraduate work in biomedical and electrical engineering at the University of Toronto. Toward the end of college, however, he became more interested in medicine and medical research, which led him to go to Johns Hopkins University for a combined M.D.-Ph.D. program, with a Ph.D in immunology.
Rabi doesn’t use his electrical engineering expertise anymore and admits that there’s a lot he forgot about, such as electrical design, but he still sees himself as an engineer. “I think the way I think about problems, even biological problems, is more in tune with how an engineer thinks about problems and problem-solving.”
One of the things that Rabi, Osho and Tessier focus on is reducing possible damage to a heart, which usually results from lack of oxygen or an extreme change in temperature. In recent years it has become possible to keep the heart colder for longer, which has allowed for hearts that were once inaccessible to reach new destinations. For example, in 2023, a heart was flown over seven hours from Alaska to Mass General Hospital in a SherpaPak container which was developed by Paragonix Technologies. In 2021, a heart was flown from Honolulu, Hawaii to Cedars-Sinai in California using the organ care system developed by Transmedics, which pushes blood through the heart and gives it oxygen.
Studying wood frogs
MGH’s Ex Vivo lab does use the SherpaPak to get a heart down to 4 degrees Celsius. But even with these advances, heart injury is possible when taking other variables into consideration. That’s why Tessier, a molecular biologist and assistant professor at Harvard Medical School, Mass General, and Shriner’s Children’s Hospital is applying what she knows about suspended animation to achieving better transplantation outcomes. Tessier has studied North American wood frogs that are able to freeze without dying and bears that hibernate for the winter.
“A lot of the features of these animals that enter suspended animation are very similar to the stresses that an organ goes through after it’s procured, during its transport and then subsequently when it’s implanted into the recipient,” Tessier said.
Tessier, 39, didn’t know that her path would lead her to working on organs, but she knew that after getting her Ph.D. which focused on animals that can survive in extreme conditions, she wanted to work on translational research for her postdoc. She said that she came to Mass General because of the “really unique ecosystem where basic science meets clinical on a daily basis.”
Looking back, she’s had the hope that one day the principles of suspended animation could be applied to transplantation since she was a master’s student. “So, I think it’s just kind of funny how I guess that ended up coming full circle. So I think I always had a natural inclination for the heart.”
Tessier sought to understand the underlying molecular pathways that enable animals to survive extreme stresses and why non-hibernators, like us humans, can’t. She does this with other models besides hearts and lungs, including cell culture, zebrafish, and small rodents. Tessier also seeks to mimic the molecular and physiological lessons learned from bears, which are able to reduce blood flow to heart while in hibernation, to extend the length of time that an organ can stay outside the body. “We want to make those perfusions days, weeks-long, so that we can keep them alive for a lot longer,” said Tessier.
Everything in the lab is touched by a problem that requires the eye of an engineer, but eventually it has to move from a problem that needs troubleshooting to a clinical intervention. “If you do want true clinical adoption, it’s got to be something that — it’s just easy to use, that can be practical,” said Tessier.
While Tessier and Rabi are working on how to perfect and simplify the system, Osho is furthering the understanding of how hearts from people who suffered a circulatory death instead of being declared brain dead can be made more viable for transplant. More specifically, there’s a period of time when the heart isn’t getting a lot of blood and that causes the death in the donor. Osho is exploring how that period can affect the function of the heart and how surgeons can prevent any excessive damage from happening before transplanting that heart into a person.
Osho, 35, believes that the work that is currently being done can change the way that DCD donation, which MGH has pioneered since 2019, is done.
“There are systems that are good, but they are expensive and they’re cumbersome and we’re working to remedy some of this,” Osho said.
Osho mirrors Rabi’s path since he originally had interest in immunology and infectious diseases. Growing up in Nigeria, he was influenced by his father who trained as a cardiologist, but also cared for all kinds of patients.
“I did some rotations in medical school and I fell in love with the operating room — by the cardiac surgery spaces. It’s a lot of technical challenge, but at the same time the physiology is just incredible,” said Osho, an advanced transplant cardiologist at Massachusetts General Hospital.
For now, the trio of researchers is focusing on making it possible for hearts to last as long as possible outside the body. In the next year or two, Rabi hopes to give supplements like metabolites for the heart to make it function properly. The team also seeks to develop treatments that will help prevent rejection and provide more flexibility during the transplantation process. The hope, said Osho, is “to change how long and how well we can preserve hearts.”
Soon, Osho said, “we’re looking at being able to do perfusions over days, which will just give us a lot more flexibility in the transplant process.”
Tessier agreed: Perhaps, she said, hearts will be able to last for days within her lifetime. And she imagines a day, perhaps not in her lifetime but soon thereafter, when there might be “a catalog of organs and a doctor can just go into a database and say, ‘Oh, my patient needs this and that and OK I’ll take that organ right off the shelf.’” Doctors will be able to search for the perfect match, and “there’s no criteria to get you on the waitlist because there’s enough organs to meet everyone’s needs.”
To submit a correction request, please visit our Contact Us page.





STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect