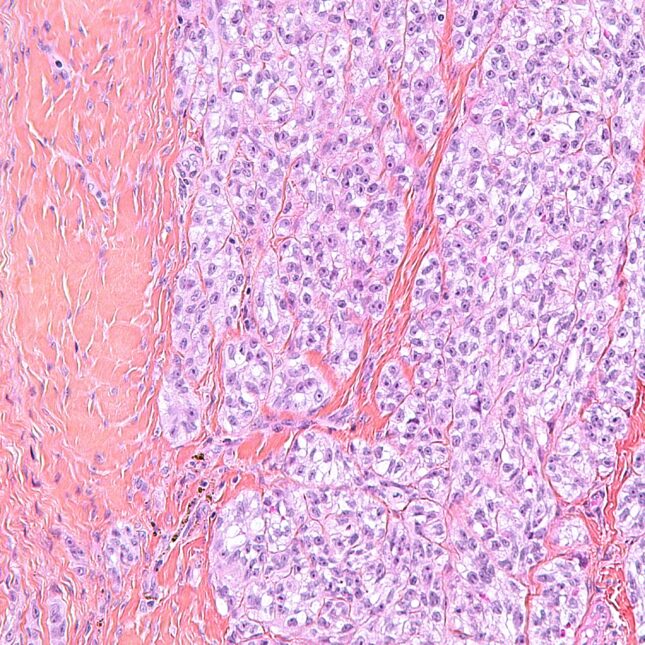
LONDON — U.S. regulators have authorized a cutting-edge treatment relying on T cells for a rare cancer that arises in the body’s soft tissues, extending the power of immunotherapies to difficult-to-reach sarcomas.
The medicine, called Tecelra and developed by Adaptimmune Therapeutics, is what’s known as a T cell receptor therapy. The approach has similarities to the CAR-T therapies that have dramatically improved the treatment of some blood cancers, but it has tricks up its sleeve that allow it to target solid tumors, which CAR-Ts have struggled to fight.
The Food and Drug Administration granted Tecelra, also known as afami-cel, accelerated approval for patients with synovial sarcomas, which can form in muscles and ligaments throughout the body, the company said Thursday night. The one-time treatment is the first authorized medicine from Adaptimmune, which has operations split between Philadelphia and Oxford in the U.K.
This article is exclusive to STAT+ subscribers
Unlock this article — plus daily coverage and analysis of the biotech sector — by subscribing to STAT+.
Already have an account? Log in
Already have an account? Log in
To submit a correction request, please visit our Contact Us page.











STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect